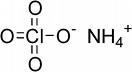What is Ammonium Perchlorate (AP)?
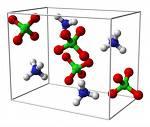
Ammonium Perchlorate (AP), with the chemical formula NH₄ClO₄, is a powerful oxidizing agent used widely in industrial, military, and aerospace applications. It is a white crystalline solid with a molar mass of 117.49 g/mol. It is moderately soluble in water and has a decomposition temperature of approximately 130°C.
Chemical Properties of Ammonium Perchlorate
| Formula: | NH₄ClO₄ |
| Molar Mass: | 117.49 g/mol |
| Appearance: | White crystalline solid |
| Solubility: | Moderately soluble in water |
| Decomposition Temperature: | Begins at ~130°C |
Ammonium perchlorate consists of the ammonium cation (NH₄⁺) and the perchlorate anion (ClO₄⁻). It is highly reactive, especially when combined with fuels or reducing agents.
Primary Uses of Ammonium Perchlorate
1. Solid Rocket Propellant
Ammonium perchlorate is a key oxidizer in solid rocket motors, particularly in missile systems and space launch vehicles such as the NASA Space Shuttle boosters. It is typically combined with a fuel like powdered aluminum and a binder (HTPB) to form a composite propellant.
2. Military Applications
Ammonium perchlorate is used in pyrotechnics, explosives, and ammunition. It also plays a role in gas generators and decoy flares.
3. Fireworks and Pyrotechnics
Although alternatives are often preferred due to environmental concerns, ammonium perchlorate is sometimes used in fireworks and other pyrotechnic devices.